Based on studies to date in both civilian and military settings, we recommend changes to the current definition of PTH. Anxiety disorders such as post-traumatic stress disorder (PTSD) are frequently associated with TBI, especially in military populations and in combat settings. PTSD can complicate treatment of PTH as a comorbid condition of post-concussion syndrome. PTH should not be treated as an isolated condition. Comorbid conditions such as PTSD and sleep disturbances also need to be treated. Double-blind placebo-controlled trials in PTH population are necessary to see whether similar phenotypes in the primary headache disorders and PTH will respond similarly to treatment. Until blinded treatment trials are completed, we suggest that, when possible, PTH be treated as one would treat the primary headache disorder(s) that the PTH most closely resembles.
Introduction
Headache is one of the most common and persistent symptoms following traumatic brain injury (TBI)[1–3] and has been particularly targeted by recent studies addressing mild TBI (mTBI, Table 1 ) in returning military personnel and athletes.[4–9] This manuscript addresses the following aspects of post-traumatic headache (PTH): (1) epidemiology of PTH; (2) features of PTH in civilian and US military populations; (3) relationships between TBI and PTH genesis; (4) interactions of comorbid conditions such as post-traumatic stress disorder (PTSD), and mood and sleep disorders with PTH; (5) our suggestions for revision of International Classification of Headache Disorders (ICHD-2) criteria for PTH.
Methods
The first draft of this manuscript, which dealt with TBI epidemiology, and post-concussion syndrome (PCS) including PTH, the relationships between TBI and development of PTH, and the interactions of comorbidities of TBI with PTH, was developed by 2 of the authors R.G.R. and R.L.R.. S.L. wrote the first draft of the section on PTH in civilians, and B.T. wrote the first draft of the section on military PTH. The database of the information for this review was developed by author R.L.R. starting from his electronic database >2700 articles on TBI in military and civilian environments. R.L.R. supplemented that database using literature searches utilizing the PubMed service of the National Center for Biotechnology Information, which uses the US National Library of Medicine as its database. He used the following Medical Subject Headings (MeSH terms) for the literature searches: "headache, post traumatic"; "tbi traumatic brain injury"; "disorders, migraine"; "secondary headache disorder"; "primary headache disorders"; "headache, tension type"; "arousal disorder, sleep"; "inflammation, brain"; "combat stress disorders"; "combat disorders"; "ptsd" and "anxiety disorders." He also performed searches for MeSH terms in article "titles and abstract." He restricted the search to articles published after January 1, 1990 and to articles written in English, French, Japanese, or German. He queried PubMed for MeSH single terms and combinations including "tbi" and "ptsd"; "headache, post traumatic" and "tbi"; "arousal disorder, sleep" and "headache, post traumatic." The PubMed searches yielded 687 additional articles to supplement the initial database. In addition to querying PubMed, he also reviewed US Government and headache society websites from the US Congress, Centers for Disease Control, National Institutes of Health, Department of Health, Department of Education Model Systems Program, Department of Defense, Department of Veterans Affairs (VA), Defense and Veterans Brain Injury Center (DVBIC), American Headache Society, International Headache Society, and European Headache Federation. Authors B.T. and S.L. developed the information in their sections utilizing their existing information databases obtained from prior studies on TBI and PTH they had done as well as ongoing studies on PTH related topics. All of the authors participated in revising the manuscript.
TBI Epidemiology
TBI is an extremely important global and national health issue. Table 1 shows TBI classification. Recent estimates for the US indicate that each year, 1.7 million civilians sustain a TBI requiring medical attention, 53,000 die, and 275,000 people are hospitalized for nonfatal TBI ( Box 1 ). About 43% of Americans have residual disability 1 year after hospitalization with TBI, and 3.2 million Americans live with disability from TBI.[11] In the US, 75% of all TBI is classified as mTBI.[11–14] The incidence of TBI requiring medical attention in Olmsted County, MN, USA was 558 per 100,000 person-years.[12] In New Zealand, 32% of people had a TBI requiring medical attention by age 25 years,[11] and the total incidence was 790 cases of TBI per 100,000 person-years with 95% of the cases being mTBI.[15] Large numbers of mTBI events do not lead to immediate medical attention, thereby underestimating the scope of persistent post-traumatic symptoms, including PTH. Falls are the most common cause of TBI among children 0–4 years old and in adults over 75 years old.[13] Civilian TBI fatalities have declined over the past 10–15 years, which may be due in part to improvements in protection of occupants in motor vehicles, protective helmet design,[16] and other preventative measures.[12, 13]
The earlier statistics do not consider the TBI burden in military personnel.[17–21] The most common cause of injury in US military personnel in the recent Middle East conflicts (Operation Iraqi Freedom [OIF]/Operation Enduring Freedom [OEF]) is exposure to combat-related explosions.[17, 18, 22] Combat TBI is distinguished from civilian TBI by the extreme physical and psychological conditions of war. The Congressional Research Service[23] reported about the US military that "Of the total 253,330 traumatic brain injury (TBI) cases between January 1, 2000 and August 20, 2012, 194,561 have been mild, 42,083 have been moderate, 6476 have been severe or penetrating, and 10,210 have not been classifiable."[23] Figure 1, derived from the DVBIC website,[24] shows TBI among US military personnel during the 21st century. Note that combat in Iraq (OIF and Operation New Dawn) was done mostly by Army personnel, whereas Marines were primarily deployed in Afghanistan (OEF). The most common cause of injury in US military personnel in the recent Middle East conflicts is exposure to combat-related explosions.[17, 18, 22] Other events possibly contributing to the disabling effects of TBI are harsh living conditions of war, inflicting irregular sleep patterns and subjecting soldiers to extreme physical and emotional demands.[25, 26] Veterans of OIF/OEF with combat-associated mTBI often have headaches, PTSD, and sleep dysfunction,[27–34] which complicate re-entry into civilian life.[25, 35]
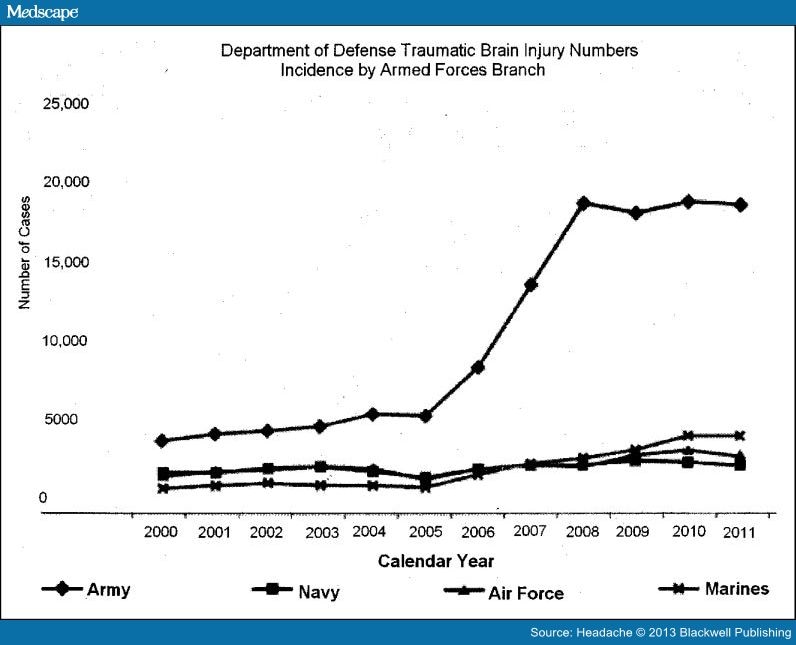
Figure 1.
The number of traumatic brain injury cases reported by each branch of the US military. The data are from the Defense and Veterans Brain Injury Center website.[24] The original source of the data was the US Department of Defense, Theatre Medical Data Store within the Defense Medical Surveillance System.
PCS
Although PTH may be the most common physical symptom following TBI, a broad range of symptoms has commonly been grouped within the PCS symptom complex ( Table 2 ). It can also include dizziness, fatigue, irritability, anxiety, impaired sleep (commonly hypersomnia or insomnia), impaired cognitive function with altered concentration and memory, blurred vision, and hyperacusis.[36]
The development and duration of PCS is not related to the severity of TBI.[31, 37] Although results have been variable with most studies indicating that those with mTBI report significantly more symptoms than people with general trauma without head injury and also those with TBI report significantly more symptoms at 1 month and/or 1 year after head injury.[3]
Controversies over the association of PCS with TBI are partly due to the non-specific nature of the PCS. In one study of 4462 randomly sampled male Vietnam era US Army veterans, only 32% with a history of mTBI met Diagnostic and Statistical Manual IV symptom criteria for PCS. In contrast, the frequencies of PCS among veterans with psychological diagnoses who did not report TBI were: PTSD 40%, generalized anxiety disorder 50%, major depression 57%, and somatization disorder 91%.[38] However, this study did not demonstrate that TBI does not induce PTH. The veterans in the previous study were evaluated more than 35 years after their TBI events. A minority of people would be expected to have chronic PCS following mTBI. Therefore, that only 32% had PCS more than 3 decades after mTBI is a reasonable expectation. This study demonstrated the non-specific nature of symptoms such as impaired memory and headache of an unspecified type. In a study of civilian trauma, people who suffered minor orthopedic injuries were as likely as those with TBI to have headaches within a month of trauma.[39] In contrast, a population study in Australia found that people who suffered mTBI > 7× more likely to have headaches 3 months after trauma compared with those who had trauma that was not TBI.[40] Hoge and colleagues[41, 42] argued that for soldiers who were deployed in Iraq during OIF, most PCS symptoms could be explained by coexistent psychological disorders such as depression and PTSD. Interestingly, the one PCS symptom that was clearly associated with mTBI even after adjustment for depression and PTSD was PTH.[41] Our opinion is that TBI is one of the possible causes for PCS, but that presence of PCS does not indicate that an individual had a prior TBI.
Additional support for PTH being associated with mTBI is that PTH correlates with the presence of neurological dysfunction on physical examination[27, 32–34] or neurophysiological testing.[43, 44] PCS includes symptoms related to cognitive impairment and impaired cognition correlates with cerebral structural or physiological abnormalities visualized by neuroimaging.[45–49]
PTH in Civilians
The literature on symptoms following TBI is extensive ( Box 2 ). Interpretation and comparison of these studies are difficult as there is much variability in case definitions and TBI type, subject selection bias, and poor and variable follow-up time. Also, many symptoms are self-reported with an absence of objective findings particularly in mTBI and may be discounted because of concern regarding secondary gain issues. Although headache is the most common symptom following TBI,[1, 50–52] the prevalence has ranged from 30% to 90% in retrospective studies with 18–22% lasting more than 1 year.[1, 53–55] Some epidemiological studies from civilian populations are listed in Table 3 . In the largest prospective study to date, 452 subjects admitted to inpatient rehabilitation services following a moderate-to-severe TBI, the majority were men injured in vehicle-related accidents with an average age of 44 years.[50] Seventy-one percent reported headache during the first year. Prevalence was 46% at the initial inpatient interview and remained high with 44% reporting headache 1 year after TBI. About 28% of headaches developed after the initial evaluation, occurring at 6 and even 12 months after injury, although most PTH developed within 3 months of injury (Figure 2). Thus, an appreciable fraction of headaches developed after the 7-day window in the current ICHD-2 criteria defining PTH.[10]
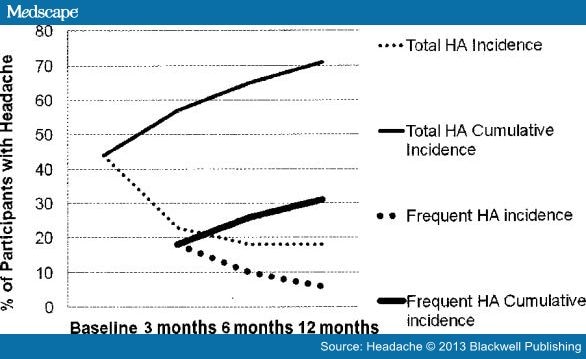
Incidence and cumulative incidence of all headaches (HA) and frequent HA occurring after TBI in a group of 452 civilians who were in an acute inpatient rehabilitation program following moderate or severe traumatic brain injury. The total HA incidence is the fraction of subjects who first developed a post-traumatic headache at each time point. This figure was modified from figure 1 of Hoffman et al50 with permission of an author (S.L.)
Similar to other symptoms of the PCS, PTH may be more frequent among individuals who seek medical attention after mTBI compared with those who have moderate or severe TBI.[36] In the first year after TBI, 71% of adults with moderate or severe TBI reported PTH.[50]Incidence of PTH was >90% among 212 individuals admitted within 1 week after mTBI.[56] Others also noted a higher prevalence of PTH following mTBI compared with more severe TBI.[53, 54, 57, 58] The factors that enhanced the likelihood of PTH were prior history of headache and female gender. Although prior history of headache was significantly related to PTH regardless of TBI severity, female gender as a risk factor for PTH was much stronger in those sustaining a moderate-to-severe TBI than following an mTBI.[50, 52] When classification criteria for primary headache disorders are used to characterize civilian PTH that persists more than 1 month after trauma, migraine or probable migraine is the most common headache phenotype.[50, 52] For PTH following moderate-to-severe TBI, migraine or probable migraine was found in over 52% of those reporting headache at baseline evaluation and 54% of those with headache at 1 year.[50, 52] Even in individuals without a prior history of headache, migraine/probable migraine was found in 62% of those reporting headache at baseline and in 53% at 1 year. Migraine/probable migraine was also the most common headache phenotype in civilians with mTBI (Lucas, Hoffman, Bell, and Dikmen, unpublished data).[51] The civilian studies of Hoffman et al[50] and Lucas et al[52] contained primarily male individuals injured in vehicle accidents. Their headaches were more likely to resemble migraine or probable migraine compared with headaches in males in the general population.[59] In the civilian groups studied by Hoffman et al[50] and Lucas et al,[52] tension-type headaches never exceeded 21% of any group at any time point regardless of TBI severity. Similarly, cervicogenic headache made up 10% or fewer of classifiable headaches at any time points.[50, 52] This is in contrast with earlier studies where tension-type headache was the most common PTH after mTBI.[39, 60, 61] Some potential factors underlying differences could be that one study was based on selected clinical samples with a long time between injury to consultation,[60] and another study was a retrospective chart review.[61] Another difference is that in the prospective studies by Hoffman et al[50] and Lucas et al,[52] classification was based upon only the most severe headache type that a subject reported at each time period so that if both migraine-like and tension-like headaches were present, migraine headaches would be the reported headache.
PTH frequency is higher in those with more intense headache pain. Comparing civilians who had PTHs that resembled migraine or probable migraine with those with PTHs that resembled tension-type headache or cervicogenic headache, those with migraine-like headaches were most likely to describe headaches occurring several days per week or daily.[52] In fact, 23% of individuals sustaining moderate-to-severe TBI reported headache frequency of >15 days of headache per month (criteria for chronic daily headache [CDH]) at all time points up to 1 year after TBI, and this rate doubled following mTBI. In contrast, 4–5% of those with headache in the general population report CDH.[62–66] Head and neck injury accounts for approximately 15% of CDH cases.[67]
In the US, about 20% of all civilian TBIs occur in amateur athletic competitions at or below the college level[11–14] and are primarily mTBI with only about 9% of cases requiring hospitalization.[36, 68–71] Although approximately 90% of athletes with mTBI are symptom-free within 1 month,[6, 71] 10–20% may continue to have symptoms of PCS including PTH.[36, 71] Athletes who sustained multiple episodes of TBI are more likely to have PTH that persists longer than 1 month.[6, 72]
Rare cases of PTH in the civilian population are phenotypically similar to cluster headaches,[73] hemicrania continua,[74] chronic paroxysmal hemicranias,[75] and short-lasting, unilateral, neuralgiform headache attacks with conjunctival injection and tearing (SUNCT syndrome).[76] Some PTH patterns are associated with specific craniocerebral injuries.[28] Leakage of cerebrospinal fluid (CSF) can produce low CSF pressure headaches. Post-craniotomy headaches can complicate post-surgical treatment of TBI. However, there has been no significant correlation between acute neuroimaging abnormalities, and presence or absence of PTH.[77]
PTH in Military Combat Settings
PTHs were an important cause of medical evacuation from Iraq or Afghanistan, and failure to return to duty ( Table 4 ). Only 18% of service members evacuated with PTHs returned to duty; this was the lowest rate among all headache types.[78] Most recent US military PTHs developed following blast exposure.[17, 19, 21, 27] A common injury scenario is an explosion-induced vehicle crash in which an individual is exposed to the blast (primary blast effect) followed immediately by a secondary injury from the vehicle crash (tertiary blast effect).[27] It is often not possible to separate the primary blast effects from secondary and tertiary blast effects. Blast exposure occurred in over 80% of 978 US Army soldiers reporting headaches after return from deployment, and on average, these soldiers were exposed to 5 or more blasts occurring within 60 feet.[79] Other studies found that OIF/OEF veterans with multiple episodes of mTBI with loss of consciousness (LOC) were more likely to have more severe PCS.[27, 32–34, 80] OIF/OEF veterans who had mTBI with LOC had more white matter abnormalities present on diffusion tensor cerebral MRI.[81]
Seventy-seven percent of soldiers with chronic PTH had blast-induced TBI.[82] Among recently deployed US Army soldiers, nearly 40% of PTH occurred within 1 week of the mTBI ( Box 3 ). Another 20% occur within 1 month, and just over 40% occurred beyond 1 month of head trauma.[21] Therefore, the majority of these headaches would not meet the current ICHD-2 criteria for PTH,[10] and PTH occurrence would have been underestimated.
Hoge et al[41] reported that among 2525 US Army infantry soldiers, PTH was the only PCS significantly associated with mTBI after controlling for depression and anxiety disorder. From 2001 to 2007, a time period coinciding with the first 6 years of OIF/OEF, the incidence rate of migraine increased in US service members, with the most marked increase in the US Army (Fig. 1).[23, 83] The prevalence of migraine appears to be markedly higher in US Army soldiers upon return from deployment with 36% of US Army soldiers in a single combat brigade reporting headaches with features of migraine.[84] In the Millenium Cohort study including over 77,000 US Army soldiers screened before and after deployment, soldiers with combat exposure had significantly increased odds of a new onset headache disorder after deployment compared with non-deployers.[85] Soldiers who deployed but did not experience combat did not have an increase in new onset headaches after deployment compared with non-deployers, emphasizing the importance of combat exposure.[85] Psychological factors may contribute to the genesis of migraine. Among men who did not have predeployment migraine, the incidence of migraine increased nearly 4 times for men with comorbid anxiety (including PTSD) or depressive disorders.[86] Mood and other factors that contribute to psychological resiliency also influence the presence and severity of PCS after combat TBI. Positive mood and high psychological resilience prior to deployment protect against persistent anxiety (including PTSD) and PTH developing after mTBI.[87]
Migraines were 5.4 times more likely after deployment in service members with a concussion during deployment.[86] Military PTH meet criteria for migraine in the majority of cases ranging from 60% to 97% depending on the study population and methodology.[20, 21, 33, 82]While migraine is clearly the predominant headache phenotype in clinical studies where additional headache types were assessed, many soldiers with PTHs have additional headache types including tension-type, craniofacial neuralgiform pain disorders, and rarely trigeminal autonomic cephalalgias.[20, 33, 88] Between 30% and 50% of US service members report using short-acting analgesics, mostly over-the-counter medications, on 15 or more days per month across the recent studies. The proportion of these patients who meet criteria for medication overuse headache is unknown.
A striking feature of the studies of headaches, especially PTHs, in the military is the high prevalence of chronic headache syndromes, particularly CDH. Among 978 US Army soldiers with deployment-related concussion, 20% reported headaches on 15 or more days per month in the preceding 3 months with a median of 27 headache days per month. Fifty-five percent of the soldiers with CDHs had their headaches start within 1 week of a concussion.[79] Similarly, among 100 US Army soldiers with chronic PTHs seen in a headache clinic, the average headache frequency was 17 days per month.[82] Migraine features are present in the majority, 70% or more, of the chronic PTH disorders in the military studies. Screening criteria for PTSD are met in 40–50% of soldiers with chronic PTHs.[79, 82] Chronic PTH patients from military or VA referral populations also tend to report poor sleep quality and nightmares.[32–34] The chronic symptomatology of service members and veterans following TBI is an overlap of chronic PTH, PTSD, and other psychiatric disorders.[28, 29, 35] Direct attribution of cognitive, psychological, and pain symptoms in the chronic post-traumatic syndrome is difficult when CDH and psychiatric disorders coexist in someone who sustained multiple concussions in combat.
The DVBIC provides treatment recommendations for management of headaches in deployed and non-deployed settings. The DVBIC and other opinion statements on PTH management are based exclusively on Class IV evidence.[24, 89, 90] In a retrospective study of chronic military PTH, triptans were particularly effective as acute agents emphasizing the predominantly migraine phenotype. Topiramate was the only prophylactic agent noted to be associated with a statistically significant reduction in headache frequency, but overall, the number of patients using different medications was small, which prevented treatment recommendations.[82] Overall, only 35% of soldiers were considered responders to prophylactic agents.[82] This high non-responder rate requires further prospective evaluation. Theeler and Erickson[90] provided treatment algorithms for different patterns of PTH seen in military personnel.
The outcomes of military personnel involved in blast-related concussions in combat and the civilian suffering a concussion following a motor vehicle accident are likely to be different. However, more similarities than differences are seen in recent military and civilian studies despite differences in trauma mechanism and circumstance, TBI severity, and methodology in these studies.[26, 27, 33, 50, 52, 82] For both civilians and military personnel, chronic pain including PTH is more likely to occur following mTBI compared with moderate or severe TBI.[37] Meta-analysis of TBI studies showed that about half of civilians or military personnel have chronic pain, usually PTH, following TBI.[37] The studies included in the meta-analysis may contain populations of subjects that overrepresents the presence of chronic post-TBI pain ( Box 4 ).
Relationships Among TBI, PTSD, and Other Psychological Disorders
The relationship between PTSD and brain injury is complex. As with PTH, PTSD more commonly develops after mTBI compared with more severe TBI.[91] A possible distinguishing feature between TBI in civilian and combat setting is the high prevalence of PTSD and sleep disorders among those with combat-acquired TBI, especially mTBI.[2, 41, 92–95] About 40% of veterans and military personnel who served in OIF/OEF and sustained mTBI from combat had PTSD.[28, 41, 96–99] Among soldiers deployed in OIF, 44% of those with an episode of LOC because of mTBI had PTSD compared with 16% of soldiers with other injuries and 9% for uninjured soldiers ( Box 5 ).[41]
Meta-analysis of both civilian and combat TBI suggests that psychological conditions such as PTSD may augment the severity of PTH.[37]PTSD is a sustained anxiety reaction that develops after an individual is exposed to a psychologically traumatic event. In civilian motor vehicle accidents (MVAs), 34.4% of individuals involved in a severe MVA, in which others were likely to be seriously injured, met diagnostic criteria for PTSD 1 month after the accident.[100, 101] Military personnel suffering mTBI in combat who have PTSD are more likely to have refractory persistent PTHs including CDH.[2, 27–29, 33, 35, 102, 103] About 30% of civilians with chronic persisting PTH have PTSD.[104]Good pain control, supportive social environment, psychological resiliency, higher levels of education, and intelligence may reduce the likelihood of an individual developing PTSD.[35, 105–108]
The presence of mTBI may enhance the likelihood that a psychologically traumatic event will induce PTSD.[27, 32, 35] In rodent models of blast-induced TBI, development of PTSD-like behaviors is seen.[109, 110] In studies of both children and adults following mTBI studies, there is higher likelihood of developing PTSD.[92, 111] While minor injury to the frontal lobes may potentiate PTSD genesis,[27, 35, 94, 112] severe bilateral anterior frontal lobe damage may prevent PTSD.[113] Similarly, while injury-induced hyperexcitability of the amygdala enhances the likelihood of PTSD,[114] obliteration of the right amygdala prevents PTSD.[113] Studies of identical twins suggest that minor alterations in brain function[94, 115, 116] in areas that influence the activity of the amygdala[112, 114] alters susceptibility to PTSD.
Pituitary adenylate cyclase-activating peptide (PACAP) and the PACAP receptor together form a molecular complex that may connect PTSD and migraine.[117, 118] PACAP regulates cellular stress response and participates in vasodilation of meningeal arteries and the trigeminovascular system,[118–123] and may be coregulated with calcitonin gene-related peptide (CGRP).[119] Infusion of PACAP dilates meningeal vessels producing migraine-like headaches in migraineurs and at higher doses in healthy subjects that can be attenuated by serotonin 5-HT1B/1D agonists.[124, 125] In women with histories of sexual and childhood trauma, the presence of PTSD and severity of PTSD symptoms correlated with PACAP serum levels and with a specific single nucleotide polymorphism in the gene encoding the PACAP receptor.[117] Thus, genetic variations in the expression levels and distribution of PACAP and its receptor could alter susceptibility to both PTSD and migraine, and perhaps provide a molecular bridge between these 2 conditions ( Box 6 ).
The Pathophysiology of TBI: Does It Explain PTH?
The physiology of TBI is complex, poorly understood, but an area of intense research activity using a varied array of model systems. As the primary symptom of TBI, PTH must be linked to mechanisms associated with TBI, but as yet, these mechanisms are speculative. Perhaps, there are common paths between changes that develop following TBI and physiological changes seen in the primary headache disorders. Possible links between TBI and migraine may occur by changes in cellular and extracellular concentrations of ions and neurotransmitter availability after TBI.[126] More recent observations of inflammation and cytokine modulation, disturbances in neuronal and microglial anatomy, and abnormalities in neurotransmitter activity have been made ( Box 7 ).
Inflammation, following non-hemorrhagic closed-head TBI, is predominantly due to activation of microglia and production of inflammatory and pro-inflammatory molecules.[127–130] In animal models, even minor TBI can activate microglia and initiate neuroinflammation.[131]More severe TBI can disrupt the blood-brain barrier leading to extrusion of neutrophils from leaky blood vessels contributing to an inflammatory cascade.[127, 128] The rapid onset of neuroinflammatory changes has been confirmed in post-mortem studies of civilians demonstrating elevated messenger RNA levels of interleukin (IL)-1β, IL-6, IL-8, and tumor necrosis factor-α within minutes after TBI.[132]
Microglia plays an important role in brain response to TBI. Decreasing microglia activation can reduce brain injury. Several microglial modulators including poly ADP ribosome polymerase 1 (PARP-1), peroxisome proliferator-activator receptor (PPAR) agonists, and metabotropic glutamate receptor 5 (mGluR5) can increase neuronal survival in animal models of TBI.[133–135]
Inflammatory mediators induced by brain injury may alter the function and excitability of meningeal and cerebrovascular structures,[136]neurons,[137–139] and glia[140] possibly leading to cortical spreading depression (CSD).[141–143] CSD may be the common link between TBI-induced and migraine-induced trigeminovascular changes.[143–147]
CGRP activation may also link migraine with PTH.[148–150] CGRP is a potent vasodilator involved in neuroinflammation and pain modulation both centrally and peripherally.[151] CGRP can be released during CSD.[152] Additionally, glia in the trigeminal ganglion release a CGRP-like peptide, procalcitonin, contributing further to neuroinflammation.[151, 152] TBI activates the transient receptor potential V1 channel that enhances CGRP release from nociceptive trigeminal ganglia neurons,[153] possibly explaining migraine-like headache following TBI.
Suggested Modifications of Criteria for PTH
Table 5 is our recommendations for revised criteria for PTH. The differences between our suggested criteria and the current PTH criteria[10] are: (1) we include PTH with immediate and delayed onset; (2) PTH is classified as episodic or chronic based upon the frequency of headaches; (3) headaches are classified based upon relationship to primary headache disorders. In the current definition of PTH, chronic refers to headaches that have persisted for >3 months after TBI.[10]
According to the ICHD-2 criteria, PTHs must appear within 7 days of TBI.[10] We suspect that the duration of the time window in part reflected a concern that a longer window would increase the number of people who would claim to have PTH for secondary gain including medicolegal issues. Table 5 employs a larger time window suggested by Theeler and Erickson.[90] We suggest the longer time window in order to include people whom we observed developed headaches more than 7 days after TBI (see sections on PTH in civilians and military PTH). Data from civilian and military populations suggest that a 7-day window following TBI underestimates the prevalence of PTH.[1, 3, 20, 21, 50, 52, 79, 80, 88–90, 154, 155] Longitudinal imaging studies demonstrate evolving brain changes occurring over years following TBI.[156] Therefore, if PTH is associated with cerebral injury, headache development and progression might be expected to occur after 1 week. As pointed out by others,[157] the incidence of migraine for a 30-year-old male is approximately 0.021% per month.[158] The spontaneous onset of migraine, in an otherwise low-risk patient population, cannot possibly account for the significant proportion of patients who develop migraine-type headaches within 1–3 months of a head trauma. Approximately 20–30% of headaches following head trauma in the recent military and civilian studies occur after 1 week and usually within 1 month of the trauma (Fig. 2).[26, 52]In the longitudinal study of PTH by Hoffman et al,[50] the cumulative incidence of headache and frequent headache steadily increased over 12 months, but incidence tended to plateau at about 3 months following head trauma. The current 7-day latency requirement for PTH[10] is particularly problematic in severe TBI, as headache assessment is usually performed after consciousness or sufficient cognitive function is regained to report headache.
There are no symptom-based criteria to describe PTH in the ICHD-2 criteria.[10] The experience of the authors is that PTH present immediately after TBI often resembles tension headaches, whereas the most severe headaches that are present more than a month after TBI more often resemble migraine. The utility of using TBI severity as part of the classification criteria is unknown and needs to be re-evaluated in light of recent data. Our experiences suggest that the current criteria of "no typical characteristics known"[10] is no longer accurate.
A significant change from early criteria would be the inclusion of symptom complexes when appropriate. Approximately 70% of PTHs possess characteristics of primary headache disorders. Up to 30% of PTHs do not meet criteria for a primary headache disorder initially, although the proportion of unclassifiable headaches appeared to decrease over time by approximately 10% over 1 year into classifiable headaches phenotypically similar to the primary headache disorders.[52] The recent military, veteran, and civilian data suggest that migraine or probable migraine is clearly the predominant PTH phenotype occurring in over half of these cases.[21, 33, 52] A minority of individuals may have other headache phenotypes including tension-type headaches and cervicogenic pain. We suggest classification of PTH based upon underlying clinical similarities with primary headache disorders such as migraine. The suggested PTH classification ( Table 5 ) may have some utility in dividing patients into similar groups for research study purposes, particularly with respect to treatment protocols.
Classification of headaches as immediate onset (within 7 days) or delayed onset (beyond 7 days but within 1–3 months) may facilitate subject inclusion into future studies. Any time criterion can be considered arbitrary because of the gap in our understanding of the underlying pathophysiology of TBI and the genesis of PTH. One consideration may be to provide a criterion for possible or probable PTH when the time criterion is not met, but a PTH is still suspected.
Our PTH classification changes the ICHD-2 3-month delineation of acute (resolves within 3 months) vs chronic (continues for greater than 3 months) PTH.[10] We suggest that classification based upon headache frequency (eg, episodic vs chronic migraine) is a better method of classification and provides information useful for treatment decisions.
The current secondary headache criteria have separate classifications for PTH and headache following whiplash injury. Other secondary headache disorders isolate specific injuries (post-craniotomy, intracranial hematoma, subdural hematoma, epidural hematoma) into separate classification schemes.[10] Whether these entities, particularly headache following whiplash injury, deserve separate classifications is unclear. To facilitate comparative studies, including a criterion for the presumed primary traumatic mechanism under a single PTH classification scheme (eg, blast, blunt trauma, whiplash, epidural hematoma, etc), may be a considered in the next iteration of the ICHD.
Conclusions
PTH is the most frequent symptom reported after TBI. PTH was frequent in both military and civilian populations. For migraine-like headaches, the most severe headache phenotypes were associated with the highest headache frequencies. PTHs persist beyond 1 year in about 20% of individuals. In both the civilian and military populations, the most frequent PTHs could be classified using primary headache disorder criteria and most frequently were classified as migraine or probable migraine. PTSD is more likely to develop after trauma that includes TBI, especially in military and veteran populations. Just as comorbid conditions can complicate diagnosis and treatment of the primary headache disorders, PTSD can complicate treatment of PTH as a comorbid condition of PCS.
Data from both civilian and military populations may support changes in the classification criteria of PTH that could promote more accurate diagnosis by including additional cases of headache following injury and also considering similarities between headache phenotypes and response to medication or other interventions that may improve the life and reduce disability in this group of people. Currently, no PTH-specific evidence-based recommendations for treatment exist.
The high incidence, prevalence, and frequency of PTH requires an evidence-based approach to treatment options for those who sustain brain injury. Double-blind placebo-controlled trials in the PTH population are necessary to see whether similar phenotypes in the primary and secondary headache disorders will respond similarly to treatment. Until blinded treatment trials are completed, we suggest that, when possible, PTH be treated as one would treat the primary headache disorder(s) that the PTH most closely resembles.
Post-traumatic Headaches in Civilians and Military Personnel
A Comparative, Clinical Review
Brett Theeler, MD, Sylvia Lucas, MD, PhD, Ronald G. Riechers II, MD, Robert L. Ruff, MD, PhD
Disclosures
Headache. 2013;53(6):881-900.